Alkali and Alkaline Earth Metals
The elements in group one of the periodic table (with the exception of hydrogen - see below) are known as the alkali metals because they form alkaline solutions when they react with water. This group includes the elements lithium, sodium, potassium, rubidium, caesium and francium. Each of these elements has just one valence electron, which means that they form only weak metallic bonds. As a result, they are relatively soft and have low melting points.
The single valence electron is easily lost, making these metals highly reactive. They react vigorously with both air and water - when sodium comes into contact with water, for example, it reacts violently to form sodium hydroxide and hydrogen. The heat of the reaction actually ignites the hydrogen! Alkali metals also readily combine with the elements of group seventeen (chlorine, fluorine, bromine etc.) to form stable ionic compounds like sodium chloride.
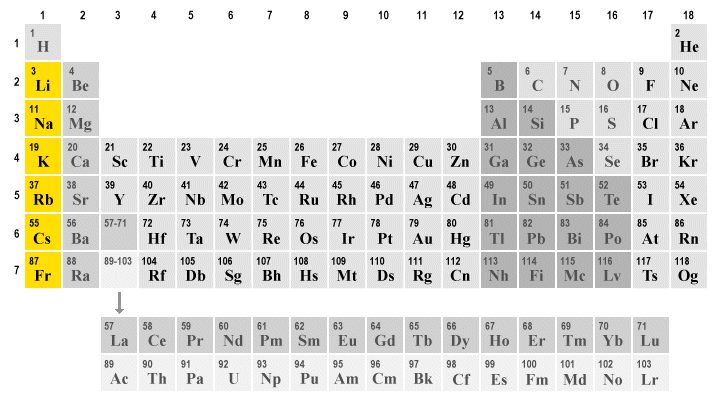
The alkali metals (highlighted) occupy group one in the periodic table
Group two of the periodic table comprises the elements beryllium, magnesium, calcium, strontium, barium and radium. The elements in this group, which are all shiny and silvery-white in appearance, are known as the alkaline earth metals. Like the alkali metals, they form alkaline solutions when they react with water. The term "earth" is historical; it was the generic name used by alchemists for the oxides of these elements (which at one time were thought to be elements in their own right).
All of the elements of group two have two electrons in their outer shell. Metallic bonds in the alkaline earth metals are thus stronger than for the alkali metals, resulting in higher melting points, but they are still quite reactive because the two outer electrons are easily lost. As a result, they are not found in nature in their elemental state.
All but one of the alkaline earth metals react with the halogens (chlorine, fluorine etc.) to form ionic compounds (beryllium chloride is the exception, because the bonding is covalent). All of the alkaline earth metals except beryllium and magnesium also react with water to produce hydrogen gas and their respective hydroxides (magnesium will react with steam, however). Essentially, the heavier the alkaline earth metal, the more vigorously it will react with water.
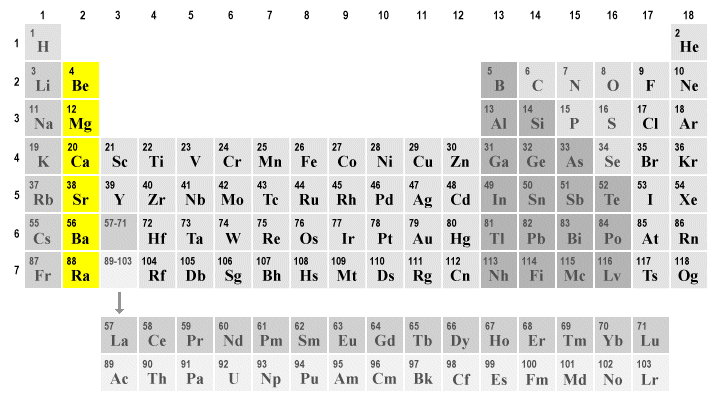
The alkaline earth metals (highlighted) occupy group two in the periodic table
Magnesium is the fifth most abundant element on earth, closely followed by calcium in eigth place - which is just as well, since both magnesium and calcium are vital to all living things, including human beings! Magnesium plays a part in a huge array of biochemical reactions; among other things, it is essential for healthy bones and teeth. Calcium makes up roughly two percent of our total body weight, with most of it residing in our teeth and bones.
