Transition Metals
The largest block of elements in the periodic table is a group known as the transition metals. These metals are found in groups three through twelve of the periodic table (the so-called d-block elements), although there are ongoing differences of opinion about exactly which elements should be classed as transition metals and which should not. Some of the most commonly used metals on Earth are transition metals, including zinc, titanium, copper and iron. Precious metals like silver and gold are also transition metals.
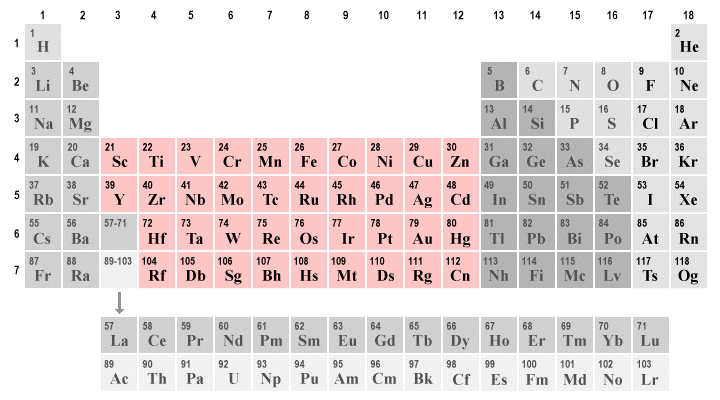
The transition metals occupy groups three through twelve in the periodic table
To complicate things further, as you can see from the simplified view of the periodic table above, there are two related groups of elements - the lanthanides and the actinides - that are usually shown separately from the rest of the periodic table. These two groups form what are often referred to as the f-block elements, and are considered by some scientists to be transition metals in their own right (they are sometimes called the inner transition metals). We will discuss these groups separately.
The definition of a transition metal can vary quite a bit, depending on who is doing the defining. One definition states that a transition metal is an element which has a partially filled d subshell, or that can be ionised to form positive ions (cations) with a partially filled d subshell. Another definition simply requires the element to be one of the d-block elements, while a third definition adds the f-block elements.
The term transition element was first coined by the English chemist Charles Bury (1890-1968), who used it to refer to what he called a transition series of elements in which an inner electron shell (for example the 3n electron shell in the elements of row four in the periodic table) was in the process of transitioning between a stable configuration of eight electrons and one of eighteen electrons, or between a stable configuration of eighteen electrons and one of thirty-two electrons.
The d-block elements belong to periods four through seven of the periodic table, and the quantum number n of the outermost electron shell of each d-block element will (by definition) be equal to the number of the period in which the transition metal resides. As we move from left to right within this group, electrons are added to the d subshell of electron shell n-1 until it is complete.
Each d-block element will have from one to ten electrons in the d orbitals of electron shell n-1, and either one or two electrons in the single s orbital of its outer electron shell (except palladium - the only d-block element not to have a 5s subshell at all). The electronic configuration of any d-block element can thus be written using the so-called noble gas configuration, and will take the following form:
[noble gas] (n-1)d 1-10 ns 0–2
The noble gas referred to will be the one in the period immediately preceding the one in which the d-block element resides. The electronic configuration of iron (Fe) could thus be written using the noble gas configuration as follows:
[Ar] 3d 6 4s 2
The transition metals typically have the following properties:
- a tendency to form coloured compounds
- good conductors of heat and electricity
- high melting and boiling points
- high density
- malleable (can be bent or hammered into shape)
- ductile (can be drawn into wires)
- relatively hard
- less reactive than alkali and alkaline earth metals
The transition metals form a bridge between the s-block elements on the left-hand side of the table, in which the valence electrons are exclusively found in s orbitals, and the p-block elements on the right-hand side of the periodic table (in which the valence electrons reside in p orbitals). The s-block and p-block elements are sometimes collectively referred to as the main block elements.
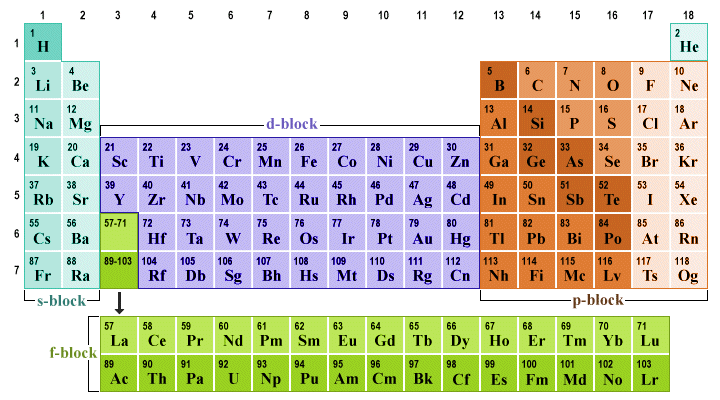
The transition metals in the d-block form a bridge between the s-block and the p-block
All d-block elements in a period n have at least one electron in their (n-1)d subshell. Most of these elements have either incomplete (n-1)d subshells or incomplete ns subshells; in some cases, both the (n-1)d subshell and the ns subshell are incomplete. The notable exceptions are the elements of group twelve (zinc, cadmium, mercury and copernicium), all of which have the noble gas configuration [noble gas] (n-1)d 10 ns 2.
The group twelve elements are often considered not to be transition metals because their outermost d and s subshells are both full. In order to understand what gives the transition metals their particular properties, however, we need to understand the significance of the incomplete d subshell and how it relates to the oxidation state of an element.
We don't want to delve too deeply into the chemistry of the transition metals and their compounds here - that is a subject to be dealt with elsewhere. Nevertheless, the electron configuration of an element obviously has a bearing on how it will behave in chemical reactions with other elements. The oxidation state of an atom represents the number of electrons the atom contributes to a particular chemical bond.
The transition metals differ from both s-block and p-block elements in that electrons in both the (n-1)d subshell and the ns subshell can be considered to be valence electrons. This sounds perfectly reasonable, given that the Madelung rule predicts that the ns orbital will fill before the (n-1)d orbitals (if you are not familiar with the Madelung rule, see the page entitled "Electron Shells and Orbitals").
There is however a slight problem here. The Madelung rule is fairly good at predicting the final electron configuration for elements in all but a handful of cases, but in terms of predicting the order in which the subshells fill, it starts to come unstuck once we get to the transition metals. Here is a diagrammatic representation of the Madelung rule, showing its predictions for the order in which the orbitals will be filled:
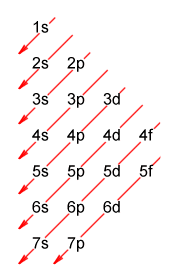
A diagrammatic representation of the Madelung rule
To find the order in which the subshells fill with electrons, follow the arrows from top to bottom and from right to left. Each entry gives a principal quantum number (which identifies the electron shell) and a letter that represents one of the orbital types (s, p, d or f). Here is the complete sequence:
1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p
The 1s subshell at the bottom is the least energetic, and fills first. Next comes the 2s subshell, and so on. The diagram thus implies that the energy level of the 4s subshell is lower than that of the 3d subshell, and that the 4s orbital will fill before the 3d orbitals. Unfortunately, this version of events is misleading. A detailed explanation of the problem is perhaps beyond the scope of this page, but we'll try and present a somewhat simplified version in the hope that it will help to clarify matters.
The Madelung rule works perfectly well for all the elements in the period table up to and including Argon (Ar). Furthermore, as we can see by looking at the electronic configuration of potassium (K) and calcium (Ca), the 4s subshell would indeed appear to be at a lower energy level than the empty 3d subshell, based on the requirement that the orbitals with the lowest energy levels are filled first. The noble gas configurations for potassium and calcium are thus [Ar] 4s 1 and [Ar] 4s 2 respectively.
The problems start when we get to the d-block elements. To cut a long story short, we know from experimental data that, during ionisation, electrons in the 4s subshell are lost before any of the 3d electrons. This would seem to indicate both that the 4s subshell fills last, and that that it has a higher energy level than the 3d subshell, which clearly contradicts what the Madelung rule tells us.
So, what exactly is going on with the d-block elements? Think about what happens as you move from left to right in the periodic table. Each time the atomic number increases by one, an additional electron is thrown into the mix, and carries a negative charge. Remember, however, that an extra proton is also being added to the nucleus, increasing its positive charge.
The interactions between the attractive and repulsive electrostatic forces within the atom are therefore going to change - and inevitably become more complex - as the number of protons and electrons increases. It is the relationship between these electrostatic forces that will determine the energy levels of individual orbitals, and the subshells to which they belong.
Scandium has the noble gas configuration [Ar] 3d 1 4s2. One electron has been added to the 3d subshell, and experimental evidence has shown that this occurs before an electron is added to the 4s subshell, which seems to indicate that the difference in energy levels between these two subshells has been reversed - and this situation is maintained for the remaining elements in the first row of transition metals. This raises an obvious question, which is why do the next two electrons added to the scandium atom go into the 4s subshell?
Essentially, introducing a second electron into the 3d subshell at this point will result in a less stable electronic configuration than placing it in the 4s subshell. Bearing in mind that the difference in energy between the 3d and 4s subshells is relatively small, the additional energy required to push the electron into the 4s subshell is more than compensated by the extra stability gained. For the same reason, the final electron to be added to the scandium atom also finds itself in the 4s subshell.
It turns out that, for most of the d-block elements, the ns subshell contains two electrons and will be filled last (there are however several exceptions). The real lesson to be learned here is that the Madelung rule is only good for predicting the final electron configuration of an element. When it comes to determining the order in which orbitals are filled, we need to look at each element on a case-by-case basis to determine exactly what is happening.
An excellent article written by Dr. Eric R. Scerri of UCLA provides a far more in-depth examination of this topic. An online version of the article, which was first published in November 2013 in Education in Chemistry, can be found here, although a subscription is required to access it. Eric Scerri's original blog on this topic "The trouble with using the aufbau to find electronic configurations", published in 2012, is also still available and can be found here.
* * *
Most transition metals have several possible oxidation states. The maximum oxidation state possible will in many cases be equal to the number of unpaired electrons in the (n-1)d orbitals, plus two (the number of electrons usually found in the ns orbital). You may recall that there are five orbitals in a d subshell, and that electrons will always occupy an empty orbital if one is available. There can therefore be a maximum of five unpaired electrons in d orbitals.
Unpaired electrons in d orbitals are less stable than paired electrons, and far more likely to participate in chemical reactions than paired electrons. Once all of the d orbitals contain a single electron, each additional electron added will reduce the number of unpaired electrons by one. Consequently, the elements with the highest maximum oxidation state - and the largest number of different possible oxidation states - will be found in the centre of the d-block, because these elements have the greatest number of unpaired electrons.
A formula for determining the maximum possible oxidation state of a d-block element could thus be written as follows:
Maximum oxidation state = no. of unpaired d orbital electrons + 2
This formula works for most of the elements in the first row of transition metals (those in period four). The notable exceptions are chromium (Cr) and copper (Cu). If chromium followed the standard pattern, it would have four electrons in d orbitals, leaving it one electron short of a half-full d subshell. It is thought that chromium "steals" one electron from the s orbital in order to acquire a half-filled d subshell. Copper exhibits similar behaviour except that, in the case of copper, the electron is "stolen" from the s orbital in order to complete the d subshell.
In subsequent rows, the formula only really applies to the elements in groups three, four, five, six and seven, due to the fact that even paired d orbital electrons can sometimes participate in chemical reactions. For the purposes of this discussion, we don't need to worry too much about the precise details.
Despite the many exceptions to the formula, the overall trend is maintained. In the second row of d-block elements, which occurs in period five, the number of possible oxidation states reaches a peak with ruthenium (Ru), which has a maximum oxidation state of +8. After that, the number becomes progressively smaller. In period six, the number of possible oxidation states peaks with iridium (Ir), which has a maximum oxidation state of +9.
The following table shows the most common oxidation states for the ten d-block elements in period four of the periodic table.
| Element | Symbol | Atomic number | Common oxidation states | Noble gas configuration |
|---|---|---|---|---|
| Scandium | Sc | 21 | +3 | [Ar] 3d 1 4s 2 |
| Titanium | Ti | 22 | +4 | [Ar] 3d 2 4s 2 |
| Vanadium | V | 23 | +2, +3, +4, +5 | [Ar] 3d 3 4s 2 |
| Chromium | Cr | 24 | +2, +3, +6 | [Ar] 3d 5 4s 1 |
| Manganese | Mn | 25 | +2, +3, +4, +6, +7 | [Ar] 3d 5 4s 2 |
| Iron | Fe | 26 | +2, +3 | [Ar] 3d 6 4s 2 |
| Cobalt | Co | 27 | +2, +3 | [Ar] 3d 7 4s 2 |
| Nickel | Ni | 28 | +2 | [Ar] 3d 8 4s 2 |
| Copper | Cu | 29 | +1, +2 | [Ar] 3d 10 4s 1 |
| Zinc | Zn | 30 | +2 | [Ar] 3d 10 4s 2 |
We have seen that electrons from the d orbitals of transition metals can be involved in chemical reactions and can thus be considered to be valence electrons. These same electrons can also be involved in metallic bonding. Because transition metals have more valence electrons than main group metals, the metallic bonding in transition metals is generally stronger than in main group metals. As a result, transition metals tend to be harder than main group metals, and have correspondingly higher melting points.
The table below shows the melting points of the main group metals and the transition metals in group four of the periodic table. You can see that the melting points of the transition metals are considerably higher than those of the main group metals, with the exception of zinc (Zn). Note also that the melting point of manganese (Mn) is lower than that of either chromium (Cr) or iron (Fe), the elements immediately to the left and right of manganese in the periodic table.
| Element | Symbol | Atomic number | Melting point |
|---|---|---|---|
| Potassium | K | 19 | 63.5 °C |
| Calcium | Ca | 20 | 842.0 °C |
| Scandium | Sc | 21 | 1541.0 °C |
| Titanium | Ti | 22 | 1668.0 °C |
| Vanadium | V | 23 | 1910.0 °C |
| Chromium | Cr | 24 | 1907.0 °C |
| Manganese | Mn | 25 | 1246.0 °C |
| Iron | Fe | 26 | 1538.0 °C |
| Cobalt | Co | 27 | 1495.0 °C |
| Nickel | Ni | 28 | 1455.0 °C |
| Copper | Cu | 29 | 1084.6 °C |
| Zinc | Zn | 30 | 419.5 °C |
| Gallium | Ga | 31 | 29.8 °C |
Manganese has both the highest maximum oxidation state and the highest number of possible oxidation states in the first row of transition metals because it has more unpaired electrons in d orbitals (five) than any other transition element in period four. As we have seen, unpaired electrons in d orbitals are more likely to participate in chemical reactions than paired electrons. So why is the melting point of manganese significantly lower than that of its immediate neighbours?
The melting point of a transition metal is to a large extent related to the strength of the metallic bonding that occurs in that metal. We can see that, in each row of transition metals, the general trend is for melting points to increase as the number of unpaired electrons in their d orbitals increases, and then fall again as the d orbitals are filled. This is because the unpaired electrons are more likely to become delocalised and participate in metallic bonding.
Manganese therefore exhibits a lower melting point than we would expect, given that it has five unpaired electrons - the maximum number possible - in d orbitals. One of the main reasons given for this seeming anomaly (although not all sources agree on the details) is that the electrons in a half-filled d subshell have a symmetrical distribution which makes the electronic configuration of the subshell more stable. Consequently, the unpaired electrons are less likely to be involved in metallic bonding.
Once the (n-1)d and ns subshells are complete, the valence electrons are even less inclined to participate in metallic bonding. The elements in group twelve of the periodic table therefore have significantly lower melting points than other transition metals (mercury is a liquid, and copernicium – which can only be created in a laboratory - is predicted to be a gas at standard temperature and pressure). For this reason, group twelve elements are often classified as post-transition metals rather than transition metals.
