| Author: | |
| Website: | |
| Page title: | |
| URL: | |
| Published: | |
| Last revised: | |
| Accessed: |
Chemical bonds are formed when some force of attraction exists between atoms, ions or molecules. If the bond involves two or more different elements, the result is a chemical compound. There are many different types of chemical bond. Some bonds are very strong, while others are relatively weak. The properties of a particular bond, together with the manner in which it is created, will depend upon the precise electronic configuration of the atoms or molecules participating in the bonding process.
The bonds that form between atoms of the same or different elements are called primary bonds, and they come in three basic flavours. The strongest forms of bonding are ionic bonding and covalent bonding. Ionic bonding occurs when an atom of a metallic element donates one or more electrons to an atom of a non-metallic element so that both atoms can achieve a stable electronic configuration, generating two oppositely charged ions in the process.
The bonds that form between two atoms of different non-metallic elements, or between two atoms of the same non-metallic element, are called covalent bonds. Here too, the result will be a stable electronic configuration for both atoms, but there is no outright transfer of electrons. The electrons participating in the bond are „shared“ by the two atoms. A bond is created between the two atoms due to the attraction between the two positively charged nuclei and the negatively charged shared electrons.
Covalent bonding does not result in ion formation, which leads to the conclusion that covalent bonds are not as strong as ionic bonds. Although this is generally the case, there are circumstances under which ionic bonds are relatively easily broken despite the force of electrostatic attraction between the participating elements. Any comparison of the relative strengths of ionic and covalent bonds should therefore be treated with a measure of caution.
The weakest type of primary bond is the bond that forms between atoms of metallic elements. Metallic bonding occurs when electrons that are only weakly bound to their parent atom achieve sufficient energy to break away and move freely between the atoms, resulting in positively charged metal ions surrounded by a „sea“ of negatively charged electrons, which acts as a kind of „glue“ holding the metal atoms together.
The intermolecular bonds that form between the molecules of a given molecular substance are weaker than any of the types of primary bond mentioned above because they do not involve the transfer, sharing, or movement of electrons. They depend instead on small differences in charge density that exist, either permanently or momentarily, within the the molecules themselves. A molecule is, by definition, a group of atoms held together by one or more covalent bonds. The kind of bond it can form with other molecules is very much dependent on the nature of those covalent bonds and and how they interact.
Regardless of their precise nature, all chemical bonds have one thing in common. They all owe their existence, directly or indirectly, to the electrostatic attractive forces that exist between the positively charged protons in the nucleus of an atom and the negatively charged electrons surrounding the atoms nearest to it.
During the nineteenth century, chemists organised the elements known at the time according to how they bonded chemically with other elements. They found that one group of elements (the elements we know today as the noble gases) tended to be found in nature in their elemental form.
The atoms of the noble gas elements did not seem inclined to combine with the atoms of other elements - or, indeed, with each other. It was eventually discovered that these same elements all had one thing in common - they all, apart from helium, have eight electrons in their outermost shell (helium is the exception because it only has two electrons in total).
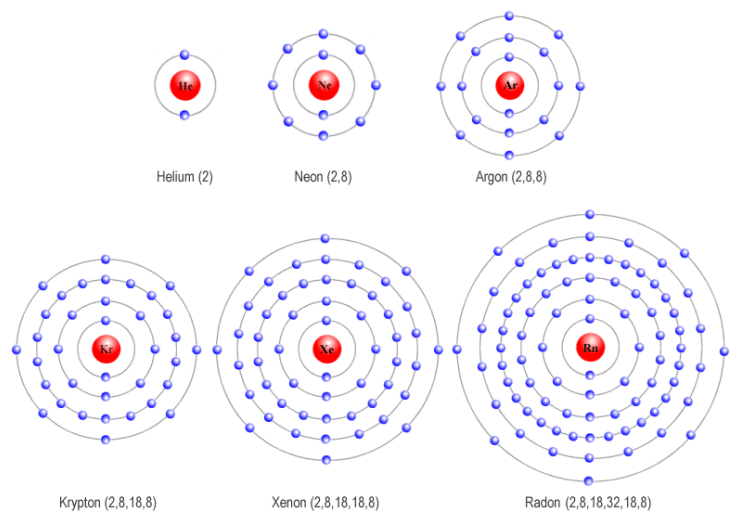
The noble gases all have outer electron shells with a stable configuration
The electrons in the outer electron shell of an atom are known as valence electrons. They have higher energy levels, and are further away from the nucleus, than other electrons. As a result, they are only weakly bound to the nucleus, and it takes relatively little energy to separate them from the parent atom. It is these valence electrons that are involved in any chemical bonding that occurs between atoms.
If you have read the article „Electron Shells and Orbitals“ in this section, you may recall that each electron shell in an atom is made up of some number of orbitals, each of which can hold a maximum of two electrons. You may also recall Hund’s rule, which states that within a given subshell, electrons will always occupy an empty orbital if one is available. Only after all of the orbitals in a subshell have one electron each will the electrons start to pair up.
Atoms with eight valence electrons have an outer electron shell consisting of one s orbital and three p orbitals, each of which contains two electrons. In this configuration, individual electrons have less energy than they would otherwise have, and are relatively tightly bound to the nucleus of the atom. This gives the atom a very stable electronic configuration.
The chemical bonding that takes place between atoms invariably involves the exchange or sharing of electrons. The atom's electronic configuration - which is defined by the number of valence electrons it has - will therefore determine how likely it is that the atom will bond with other atoms. As a general rule, the atoms of all elements apart from the noble gases have the potential to bond with other atoms.
Atoms with only one or two valence electrons have a tendency to give up those electrons when they form a chemical bond with another atom in order to achieve a stable outer shell. Conversely, atoms that require only one or two valence electrons in order to achieve a noble gas configuration tend to form bonds with other atoms that allow them to acquire the missing electrons.
A typical chemical reaction involves a process of bonding between two or more atoms in which each atom achieves a stable electronic configuration. The resulting molecule or formula unit also has a stable electronic configuration, and the energy levels in the participating atoms is lower than it would be for those atoms in their unbound state.
According to Hund’s rule (see above) an electron within a given subshell will only share an obital with another electron if no empty orbitals are available within that subshell. This behaviour is significant because each electron has a magnetic dipole moment. Two electrons occupying the same orbital will have opposite spin, so their magnetic dipole fields act in opposing directions and cancel each other out. This will not be the case for an unpaired electron. An atom with unpaired electrons thus acts as a magnetic dipole, and will interact with a magnetic field (it is said to be paramagnetic).
Since unpaired electrons are only found in an atom’s outermost electron shell, then by definition all unpaired electrons are valence electrons. However, the reverse is not true. Not all valence electrons are unpaired. For an element in its ground state (i.e. the state in which the electrons are in their lowest possible energy configuration within the atom) we can determine the number of unpaired electrons by looking at the element’s electronic configuration.
Let’s take oxygen as an example. Oxygen has the electronic configuration 1s 2, 2s 2, 2p 4 (or [He] 2s 2, 2p 4 using noble gas configuration). We thus have two electrons each in the 1s and 2s subshells, and 4 electrons in the 2p subshell - eight electrons in total, of which six belong to the atom’s outer (2n) shell and are thus valence electrons. We can show this in shorthand form using an orbital box diagram as shown below. As you can see from the diagram, two of the three 2p orbitals only have one electron, which means that the oxygen atom has two unpaired electrons.
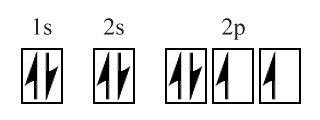
A box diagram showing the electron configuration of an oxygen atom
Substances that consist of atoms or molecules that have unpaired electrons are collectively referred to as free radicals. Unpaired valence electrons can be visualised as „dangling“ covalent bonds. With a few exceptions, substances that have one or more unpaired valence electrons are highly chemically reactive, and for that reason they are not often encountered in isolation, although low concentrations may exist in a stable condition in a vacuum, or when sharing an environment with non-reactive substances.
In larger concentrations, even in the absence of other reactive elements, atoms of elements that are free radicals will often spontaneously combine to form diatomic or even polyatomic molecules. There are seven naturally occuring diatomic elements - hydrogen (H2 ), nitrogen (N2 ), oxygen (O2 ), flourine (F2 ), chlorine (Cl2 ), bromine (Br2 ) and iodine (I2 ). The last four elements belong to the halogen family, each of which has seven valence electrons including one unpaired electron. Hydrogen also has one unpaired electron, while nitrogen has three unpaired electrons and oxygen, as we have seen, has two.
Covalent bonding occurs when two or more atoms share electrons in order for each atom to achieve a stable electronic configuration. Covalent bonds can be formed between atoms of the same type, or between atoms of different types. The simplest example of covalent bonding occurs when two hydrogen atoms combine to form a hydrogen molecule.
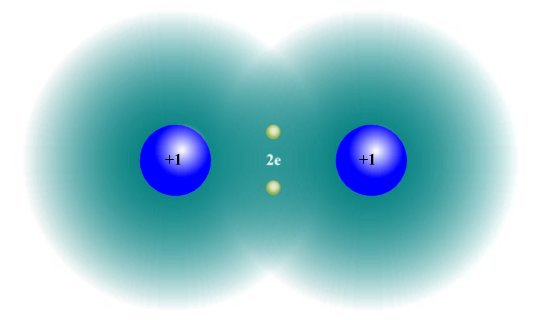
In a hydrogen molecule, two hydrogen atoms share their electrons to form a covalent bond
In a hydrogen (H2 ) molecule, the negatively charged electron in each hydrogen atom is attracted to the positively charged proton in the nucleus of the other hydrogen atom. The hydrogen atoms are sufficiently close together that their electron clouds overlap. Each hydrogen atom thus effectively gains the second electron it needs in order to achieve a full valence shell.
We saw above that a number of elements from the halogen family also have a tendency to form diatomic molecules. The diagram below shows how two chlorine atoms combine to form a diatomic chlorine molecule. One electron from each chlorine atom is shared in a covalent bond, giving both atoms a complete outer electron shell.
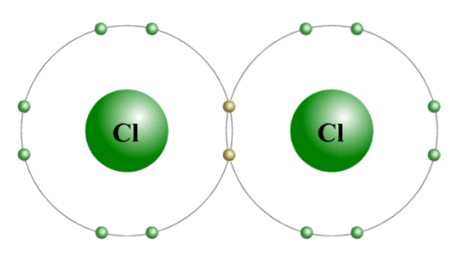
A diatomic chlorine (Cl2 ) molecule
As we mentioned earlier, when two atoms are covalently bonded, it is the attraction between the negatively charged shared electrons and the positively charged nuclei in each atom that creates the covalent bond. In order for a covalent bond to form, the nuclei of the two atoms involved in the bonding process must be sufficiently close to one another to allow their electron clouds to overlap.
As the distance between the nuclei is reduced, the electrostatic attraction between the nuclei of one atom and any valence electrons belonging to the second atom will increase. At the same time, the electrostatic repulsion between the two nuclei - and between any shared electrons - will also increase.
When the two nuclei are a certain optimum distance apart, these attractive and repulsive forces will cancel each other out and the potential energy of the system will be at a minimum. This distance is called the bond length. For the hydrogen (H2 ) molecule, the bond length is 74 picometres (74 × 10 -12 m) or 0.74 Å (one angstrom = 10 -10 metres). For a system comprising two atoms, the difference in potential energy between two atoms involved in a covalent bond and the same two atoms separated by an infinite distance is called the bond energy.
The covalent bonds we have seen so far have been single covalent bonds formed by the pairing of two single electron, one from each atom. Sometimes, two electrons from each atom are shared in a double covalent bond (this is the kind of bond found in oxygen molecules and carbon dioxide molecules). There are even occurrences of triple covalent bonds being formed, in which three pairs of electrons are shared (for example, in nitrogen molecules). Generally speaking, double covalent bonds are shorter and stronger than single covalent bonds, and triple covalent bonds are shorter and stronger than either single or double covalent bonds.
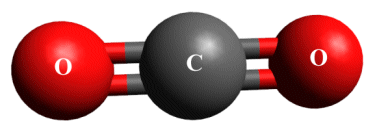
Double covalent bonds in a carbon dioxide molecule
In the carbon dioxide molecule illustrated above, each oxygen atom forms a double covalent bond with the central carbon atom. Each bond consists of two pairs of electrons - one pair from the carbon atom and one pair from one of the oxygen atoms. Each oxygen atom has 6 valence electrons, while the carbon atom has 4 valence electrons. The sharing of electron pairs in these double covalent bonds enables both the carbon atom and the two oxygen atoms to achieve a stable noble gas configuration, since each atom will have eight electrons in its outer shell.
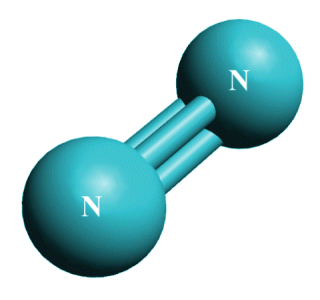
A triple covalent bond in a nitrogen (N3) molecule
In the nitogen (N2 ) molecule (above), two nitrogen atoms are bound together by a triple covalent bond. Nitrogen has 5 valence electrons, so in order for each nitrogen atom to achieve a noble gas configuration with 8 electrons in its outer shell, 3 valence electrons from each atom must be shared.
A single covalent bond between two atoms requires the sharing of two electrons, one from each atom. Each pair of shared electrons is called a bonding pair, and the number of bonding pairs making up a chemical bond between two atoms is known as the bond order. A single covalent bond has a bond order of one. Double and triple covalent bonds have bond orders of two and three respectively. Note that molecules with bond orders of four (quadruple), five (quintuple) or even six (sextuple) do exist, but are rarely encountered and as such will not be discussed here.
Several conceptual models exist that attempt to explain the nature of covalent bonding. Lewis structures are probably the simplest method available, and can be used to model the way in which valence electrons are arranged around individual atoms within a molecule using simple and easy to understand diagrams. The geometry of a molecule can be predicted using a technique known as Valence Shell Electron Pair Repulsion (VSEPR) theory, which is based on the assumption that the atoms in a molecule will arrange themselves in a way that minimises the repulsion between their valence electrons. We will be looking at both Lewis structures and VSEPR in more detail elsewhere in this section.
Valence Bond Theory (VBT) is a somewhat more sophisticated approach that attempts to explain electron sharing in terms of overlapping and hybridised atomic orbitals. Molecular Orbital Theory (MOT) describes the structure of molecles using principles of quantum mechanics, and as such presents a significant challenge to students of chemistry. Proponents of MOT claim that it predicts the properties and behaviours of molecules better than VBT, although we are inclined to the view that they should be seen as complimentary rather than competing theories. An in-depth examination of VBT and MOT is beyond the scope of these pages, but we will attempt to summarise the key concepts of each theory, and how they differ, elsewhere in this section.
Covalent bonding occurs in a number of elemental substances. In some elements, the covalent bonds can have a number of different configurations. As a result, different forms of the same element are created that can have very different properties. These variants are known as allotropes. Allotropism is the the term used to describe the ability of some chemical elements - carbon, for example - to exist in two or more different forms in the same physical state (i.e. as a solid, liquid or gas).
The element carbon has four electrons in its valence shell. In order to achieve a stable configuration, it must somehow either gain four electrons or lose four electrons. Losing four electrons completely, or gaining four additional electrons, would leave the carbon atom with a very strong negative or positive charge, either of which would leave it in an unstable state, despite having a complete outer electron shell. Sharing electrons through covalent bonding solves this problem.
Well known allotropes of the element carbon include diamond and graphite. These allotropes have very different properties. In diamond, each carbon atom shares its four outer electrons with four other carbon atoms to create a crystalline structure like the one illustrated below.
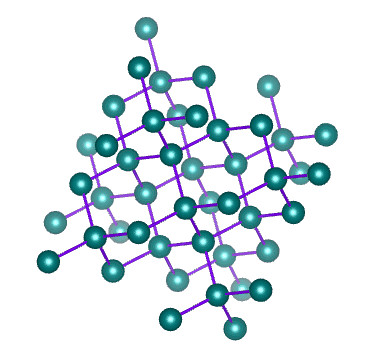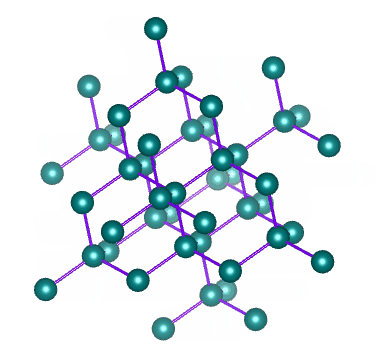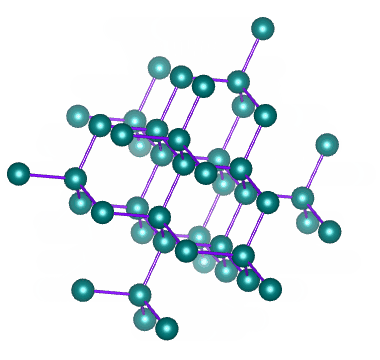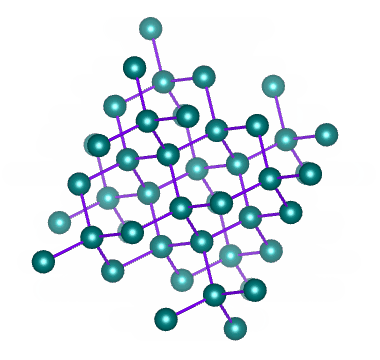
The crystalline structure of diamond – an allotrope of carbon
The animation above was created using VESTA (Visualization for Electronic and STructural Analysis), a software package developed by Koichi Momma and Fujio Izumi that is distributed free of charge for academic, scientific, educational, and non-commercial use.
As we have seen, covalent bonds can only form if the atoms involved are close enough together to share the electrons in their outer shell. Diamonds are created when a number of carbon atoms each form four covalent bonds with four neighbouring carbon atoms - something that can only occur at extremely high temperature and pressure. Most naturally occurring diamonds were formed deep within the earth's mantle.
In diamond, all of the electrons in the carbon atom's outer shell are involved in covalent bonds. There are therefor no free electrons available to facilitate the conduction of electric current (which is, after all, the movement of free electrons). Diamond therefore does not conduct electricity, unlike another more common allotrope of carbon - graphite.
In graphite, a carbon atom forms three covalent bonds with three other carbon atoms. As a result, graphite essentially forms as a two-dimensional network of carbon atoms just one atom thick, totally unlike the crystalline structure of diamond. A sample of graphite consists of many layers of these two-dimensional networks, often referred to as graphene sheets.
Within each graphene sheet, the carbon atoms are held together tightly by strong covalent bonds, but the bonds between individual graphene sheets consist of relatively weak electrostatic (van der Waals) forces. The sheets can therefore slide over one another relatively easily. This property makes graphite useful as a lubricant. Graphite is also the stuff from which pencil lead is made!
In graphite, only three of the four electrons in the carbon atom's outer shell are involved in covalent bonding; the fourth electron is free to move around between the graphene layers. This makes graphite an excellent conductor of electricity, unlike diamond.
Carbon also forms covalent bonds with other elements - in fact, all forms of life on our planet are based on carbon! One very common carbon compound is methane (CH4 ), which is formed when one carbon atom forms covalent bonds with four hydrogen atoms. Each hydrogen atom needs one electron to complete its outer shell, while carbon requires four electrons.
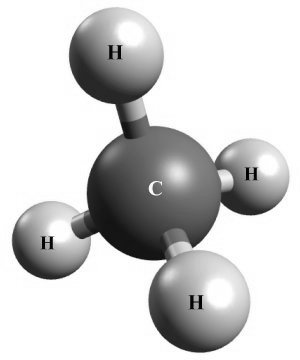
Four hyrdrogen atoms bond with one carbon atom to form methane
The polarity of a covalent bond is an important characteristic in the context of intermolecular bonding because, as we have previously indicated, the kind of bond a molecule can form with other molecules is partially dependent on the polarity of the covalent bonds holding the molecule together. The polarity of a particular covalent bond depends on the relative electronegativities of the atoms participating in the bond (we will be looking at the topic of electronegativity in more detail elsewhere in this section).
In the diagrams used to illustrate covalent bonding between two atoms, the shared electrons are often shown occupying positions located between the two atoms (the hydrogen and chlorine molecules we saw earlier are good examples). In reality, however, the shared electrons are moving at high speed and could be found anywhere around either nuclei at any given moment.
In some covalent bonds, the electrons will be more strongly attracted to the nucleus of one atom than the other, and consequently will spend more time around that atom's nucleus. Covalent bonds in which this kind of behaviour occurs are called polar covalent bonds, because the atom around which the shared electrons spend more of their time has a greater electron affinity (electronegativity) than the other atom involved in the bond.
The water molecule (H2 O) is a good example of polar covalent bonding. In water, covalent bonds form between each of the two hydrogen atoms and the single oxygen atom, but the positively charged protons in the nucleus of the oxygen atom have a far greater attraction for the shared electrons than the single proton in either of the two hydrogen atoms. The shared electrons therefore spend more time around the nucleus of the oxygen atom than they do around the nuclei of the two hydrogen atoms.
The net effect of this polarisation is to give the oxygen end of the molecule a slightly negative charge and the hydrogen ends a slightly positive charge, even though the molecule is electrically neutral overall. The molecule effectively becomes a dipole (a dipole is a pair of electric charges of equal magnitude but opposite polarity, separated by a small distance).
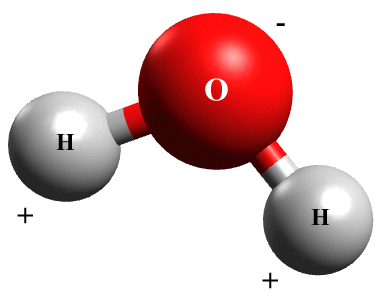
A water molecule has two polar covalent bonds
The negative and positive charges created by the asymmetric distribution of electrons in polar covalent bonds are referred to as partial charges (or net atomic charges) because they have a non-integer value when measured against elementary charge units. They are usually represented using the Greek lowercase letter 𝛿 (delta), i.e. as either 𝛿− or 𝛿+. Note that although the difference in electronegativity between the bonded atoms creates partial charges at each end of the bond, it does not change the molecule’s overall charge.
To recap, a dipole is a pair of electric charges of equal magnitude but opposite polarity, separated by a small distance. The dipole moment is a measure of this separation of charge. We can think of it as a vector quantity. The magnitude of the vector is calculated as the charge multiplied by the distance between the charges. The direction of the vector is from the negative charge to the positive charge. Dipole moment is thus calculated as:
μ = q · r
where μ is the dipole moment in coulomb metres (C·m), q is the magnitude of the charge in coulombs (C), and r is the distance between the charges in metres (m). However, because the magnitude of the charges is very small, the dipole moment is often expressed in terms of the Debye (D). The unit is named after the Dutch-American physicist Peter Joseph William Debye (1884 - 1966), who studied the charge distribution in asymmetric molecules and formulated the concept of dipole moments.
One Debye is approximately 3.33 × 10 -30 C·m. A typical polar molecule has a dipole moment in the order of 1 D. To give an example, the dipole moment of a hydrogen chloride molecule is 1.1086 D according to the CRC Handbook of Chemistry and Physics 97th Edition (2016), which gives dipole moment values for over 800 molecules obtained from various sources. All values were arrived at either using various high-resolution spectroscopic techniques or by measuring the dielectric constant in the gas or liquid phase.
Note that the values published for dipole moments in different sources (including different editions of the CRC Handbook) can vary. The most recent version of the CRC Handbook at the time of writing is the 103rd edition, which was published in July 2022. The table below shows the dipole moment values in debyes, taken from a variety of sources, for various chemical compounds. Note also that dipole moment is dependent on temperature, which may explain why different sources sometimes give different values for the same substance.
| Substance | Formula | μ/D | Description |
|---|---|---|---|
| Carbon Dioxide | CO2 | 0 | Inorganic gas |
| Methane | CH4 | 0 | Organic gas |
| Ethane | C2 H6 | 0 | Organic gas |
| Benzene | C6 H6 | 0 | Organic liquid |
| Propane | C3 H8 | 0.083 | Organic gas |
| Carbon Monoxide | CO | 0.10980 | Inorganic gas |
| Hydrogen Chloride | HCl | 1.1086 | Inorganic gas |
| Chloroform | CHCl3 | 1.15 | Inorganic liquid |
| Ammonia | NH3 | 1.4718 | Inorganic gas |
| Hydrogen Peroxide | H2 O2 | 1.573 | Inorganic liquid |
| Ethanol | C2 H5 OH | 1.69 | Organic liquid |
| Methanol | CH3 OH | 1.70 | Organic liquid |
| Hydrogen Fluoride | HF | 1.826178 | Inorganic gas |
| Water | H2 O | 1.8546 | Inorganic gas |
| Acetone | (CH3 )2 CO | 2.88 | Organic liquid |
| Nitrobenzene | C6 H5 NO2 | 4.22 | Organic liquid |
| Magnesium Oxide | MgO2 | 6.2 | Ionic solid |
| Sodium Chloride | NaCl | 8.5 | Ionic solid |
All of the substances listed in the table are molecular substances except for the last two, which are ionic compounds. Substances are listed in ascending order according to their dipole moment value. Molecules having a non-zero dipole moment can be considered to be polar molecules, although the degree of polarity will vary in proportion to the value of the dipole moment. In addition to considerations related to electronegativity, two other factors that affect the polarity of a molecule are size and geometry.
In ionic bonding, as in covalent bonding, electrons have a role to play in giving the participating atoms a complete outer shell. Unlike in covalent bonding, however, the electrons in an ionic bond are not shared between two atoms. Instead, an electron is transferred from one atom to the other.
The atom that gives up the electron becomes a positively charged ion known as a cation. The atom that receives the electron becomes a negatively charged ion called an anion. The resulting chemical bond is due to the electrostatic attraction that the oppositely charged ions have for one another.
Ionic bonding usually occurs between atoms of elements from groups one and eighteen of the perodic table. Elements in group one, such as sodium and potassium, have just one valence electron. Giving up this electron will leave them with a full outer electron shell.
Group eighteen elements like chlorine and fluorine, on the other hand, have seven valence electrons. They must therefore acquire one additional electron in order to achieve a full outer electron shell.
Chemical reactions between the (highly reactive) elements of these two groups result in stable chemical compounds like sodium chloride (common salt), a crystalline compound created when equal numbers of sodium and chlorine atoms combine. The sodium atoms become positively charged sodium ions, while the chlorine atoms become negatively charged chloride ions.
Note that within the resulting crystalline structure, the positive and negative ions are not arranged in pairs. Instead, each ion is surrounded by ions of the opposite charge in a three-dimensional crystal lattice. In sodium chloride, for example, each sodium ion (Na +) is surrounded by six chloride (Cl -) ions. Similarly, each chloride ion is surrounded by six sodium ions.
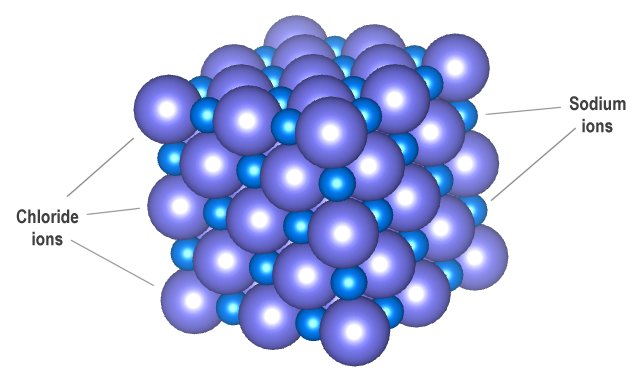
Sodium and chloride ions form a three-dimensional crystal lattice
Elements that have seven valence electrons are more electronegative than other elements (electronegativity is the ability of an atom to pull electrons away from other atoms). They are therefore more likely to gain an electron and become negatively charged. The opposite is true of elements that only have one valence electron, like sodium. They are less electronegative than other elements, and therefore more likely to lose an electron and become positively charged.
Although ionic bonds are strong, they are not as strong as covalent bonds, and can be easily broken under the right circumstances. Ionic compounds tend to dissociate (split into their constituent ions) in water, for example, because the positive and negative ions are more strongly attracted to the negative and positive poles of the water molecules (water molecules are polar - see below) than they are to each other.
Metallic bonding, as the name suggests, is the kind of bonding that usually occurs in metals. Metallic bonding occurs between the atoms of metallic elements to form metallic solids. It is different to both covalent bonding (in which electrons are shared between two atoms), and ionic bonding (in which one atom donates electrons to another atom).
In a metal, one or more electrons in each atom have sufficient energy to break away from their parent atom. This results in the creation of positively charged metal ions that are surrounded by a kind of "sea" of negatively charged electrons. These free, or delocalised, electrons are shared between the metal atoms. They hold the ions together, a bit like glue.
Although the negatively charged delocalised electrons are attracted to the positively charged metal ions, a single delocalised electron is not associated with one particular atom. In fact, at any one time, it can be associated with a significant number of metal ions. This metallic bonding is what gives metals their unique properties, which we will talk about elsewhere in this section.
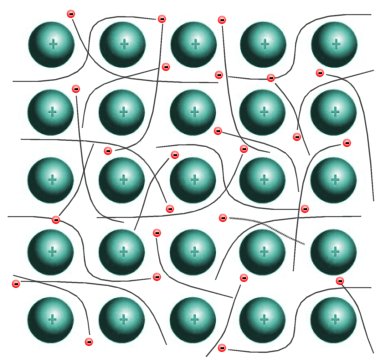
A metallic bond is a lattice of positive ions immersed in a "sea" of electrons
The strong chemical bonds that form between atoms of the same or different elements to create molecules or formula units of an element or chemical compound are called primary bonds. Covalent bonds, ionic bonds and metallic bonds are all types of primary bond.
The intermolecular forces that attract two or more molecules to one another are called secondary bonds, or Van der Waals forces after the Dutch theoretical physicist Johannes Diderik van der Waals (1837-1923), who made significant contributions to modern molecular science. Secondary bonds are significantly weaker than primary bonds. Several types of secondary bond have been identified. They include:
Note that some texts assert that the hydrogen bond does not conform to the strict definition of a Van der Waals force due to its partially covalent nature. However, it is essentially a very strong dipole-dipole interaction, so we will ignore the distinction for the purposes of this discussion.
Unlike the primary bonds that hold the atoms of individual molecules together, secondary bonds do not involve the sharing of valence electrons. All intermolecular forces, whether attractive or repulsive, are electrostatic in nature, and are the result of a positive or negative electrical charge in one molecule interacting with a positive or negative electrical charge in another molecule.
The way in which charge is distributed within an individual molecule will play a large part in determining how that molecule interacts with other molecules, and the charge distribution within a molecule depends in turn on the nature of the primary bonds holding the molecule itself together. In this sense, molecules can be broadly categorised as being polar or non-polar.
London dispersion forces are the weakest of the intermolecular forces we will examine. They are attractive forces that occur between the positive and negative ends of instantaneous dipoles. These dipoles arise because the electrons in the valence shell of an atom are not always evenly dispersed throughout the atom's electron cloud.
Over a period of time, the valence electrons are equally dispersed around the nucleus of the atom. At any given instant, however, the distribution of valence electrons throughout the cloud is asymmetric, creating a small charge imbalance. This charge imbalance can induce a dipole in a neighbouring atom if it is close enough, causing a momentary dipole-dipole attraction between the two atoms. If the atoms happen to belong to different molecules, a temporary intermolecular bond is created.
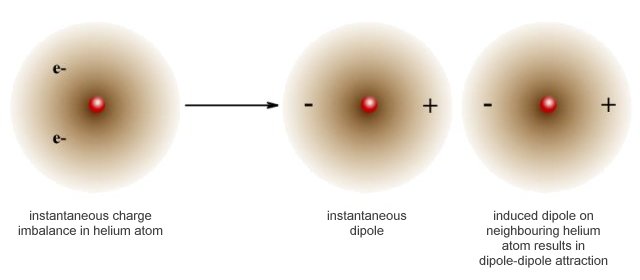
London dispersion forces create temporary dipole-dipole attractions between neighbouring atoms
Because the distribution of valence electrons within an atom is constantly changing, the distribution of electrons within a molecule will also vary over time, creating weak intermolecular bonds between neighbouring molecules. The intermolecular bonds that form between neighbouring molecules due to London dispersion forces are extremely short lived, but they can have significant consequences, not only for molecular substances.
Even a noble gas like argon, which has a full outer electron shell, will condense into a liquid if you cool it down sufficiently. As a gas cools down, its kinetic energy decreases. At some point, there will not be enough kinetic energy to overcome even the weak bonds created by the London dispersion forces, which are then able to bind the atoms or molecules together in a liquid state. If the liquid is further cooled, its kinetic energy will continue to decrease, and the dispersion forces will eventually cause it to freeze and become a solid.
The degree to which an atom can be polarised by London dispersion forces (called its polarisability) increases with atomic size because, in larger atoms, the valence electrons are not so tightly bound to the nucleus and are thus better able to form temporary dipoles. That's why argon condenses at a much higher temperature (-186 °C) than helium (-272 °C), even though both elements are noble gases.
London dispersion forces are often the only attractive forces between non-polar molecules like those of the hydrocarbons. Furthermore, because the forces are weak and of short duration, two molecules must be in very close proximity for them to have any effect. The strength of the intermolecular bonds created by the dispersion forces is also directly proportional to the surface area over which they can operate, which is dependent on the shape and size of the molecule.
Let’s consider an example. Although the hydrocarbons neopentane and n-pentane both have the same chemical formula (C5 H12 ), they have different molecular structures, as illustrated below. The n-pentane molecule has a larger surface area, allowing the London dispersion forces to create stronger intermolecular bonds between neighbouring n-pentane molecules. As a result, n-pentane has a significantly higher boiling point then neopentane.
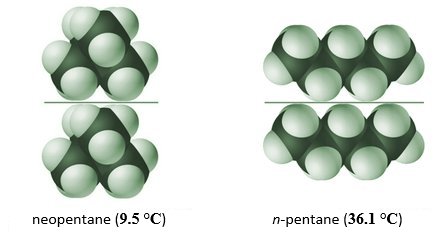
The strength of intermolecular bonds depends on shape and surface area
As a general rule, the strength of the intermolecular bonds created by dispersion forces, together with the boiling point of a substance, increases in proportion to the size of its molecules. Small molecules like methane (the main component of natural gas) experience weak intermolecular forces and thus have low boiling points. Methane has a boiling point of −161.49 °C. Larger molecules like octane (an important component of petrol), have much higher boiling points. Octane has a boiling point of between 125.1 °C and 126.1 °C.
Although we have made the distinction between polar and non-polar molecules with respect to the predominant type of bonding that occurs between them, it is worth remembering that the electrons within any atom, and thus within any molecule, are constantly moving around. London dispersion forces are thus present in all molecules, regardless of whether those molecules are polar or non-polar.
The resulting temporary dipoles can interact both with each other and with permanent dipoles. Indeed, temporary dipoles can be induced in a non-polar molecule due by a partial charge in a polar molecule, resulting in dipole-induced dipole interactions whereby the induced dipole in the non-polar molecule forms a temporary dipole-dipole bond with the permanent dipole of the polar molecule.
We saw above that covalently bonded atoms share electrons in order to achieve a full outer electron shell. If the atoms taking part in the bond are not equally electronegative, we have a polar covalent bond in which the shared electrons spend most of their time around the nucleus of the most eletronegative atom. As a result, the molecule created by the bonding process becomes polarised. One end of the molecule has a small negative partial charge, and the other end has a small positive partial charge.
Molecules that contain partial charges are called polar molecules. They are electrically neutral overall, but the separation of charge within the molecule effectively turns it into a dipole, like the water molecule we saw above. If two polar molecules are close enough together, the positive end of one of the molecules will be attracted to the negative end of the other molecule.
A good example of dipole-dipole intermolecular bonding that does not involve hydrogen bonds can be found in dichloromethane (CH2 Cl2 ), a colorless and volatile liquid that is used as a solvent in a number of chemical processes, and as a propellant in aerosol sprays. A dichloromethane molecule contains no nitrogen, oxygen, or fluorine atoms, so hydrogen bonding cannot occur (see below). It consists of a single carbon atom, covalently bonded to two hydrogen atoms and two chlorine atoms, as shown in the illustration below.
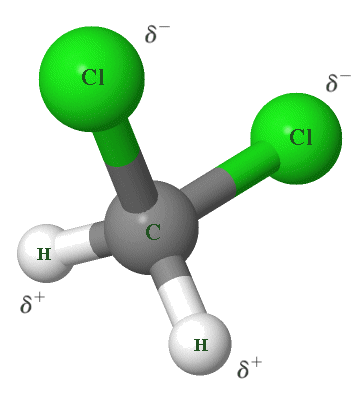
A dichloromethane (CH2 Cl2 ) molecule
As we have previously mentioned, the separation of charge in a single covalent bond - its dipole moment - is created by differences in the electronegativity of its constituent atoms. Determining the overall dipole moment of a molecule is a somewhat more complex, and will require an examination of several factors, the most significant of which are the electronic configuration of the molecule and its geometry.
The covalent bonds within a dichloromethane molecule are polar due to the significant differences in electronegativity between its constituent atoms. Specifically, chlorine has an electronegativity of 3.16, compared with 2.55 for carbon and 2.20 for hydrogen, a difference of 0.61. In order to qualify as a polar covalent bond, a difference in electronegativity of between 0.4 and 1.8 is required, so the carbon-chlorine bonds are polar and have a significant dipole moment (each chlorine atom carries a negative partial charge, while the carbon atom carries a positive partial charge).
If the dichloromethane molecule were geometrically symmetrical, i.e. with the two chlorine atoms positioned on opposite sides of the central carbon atom, these dipole moments would effectively cancel each other out. This is not the case, however. As you can see from the illustration, dichloromethane has an asymmetrical tetrahedral geometry.
The negative partial charges carried by the chlorine atoms on one side of the molecule and the positive partial charges carried by the hydrogen atoms on the other side results in the formation of a permanent dipole. The dipole-dipole interaction between two dichloromethane molecules occurs because of the attraction between the positive partial charge on one of the molecules and the negative partial charge on the other.
A hydrogen bond is essentially a special case of the dipole-dipole interaction. A hydrogen bond occurs when a hydrogen atom that is covalently bonded to a highly electronegative atom such as nitrogen, oxygen or fluorine (this atom is known as the proton donor) is attracted to another highly electronegative atom (known as the proton acceptor) nearby. A hydrogen bond is thus the result of the electromagnetic attraction between the positive charge on the proton donor’s hydrogen atom and the negative charge on the proton acceptor. Hydrogen bonds can occur both between molecules and within a single (complex) molecule.
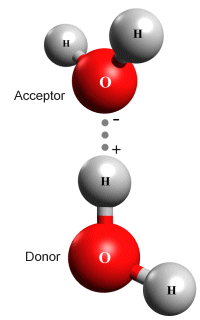
A hydrogen bond between two water (H2 O) molecules
The illustration above shows a hydrogen bond between two water molecules. In a water molecule, each hydrogen atom's electron is strongly attracted to the highly electronegative oxygen atom. As a result, it spends far more time around the nucleus of the oxygen atom than it does around the nucleus of the hydrogen atom. Each hydrogen atom thus carries a net positive charge, while the oxygen atom carries a net negative charge.
This difference in charge means that water molecules are dipoles, and we could therefore expect polar bonding to take place between them. Remember, however, that the hydrogen nucleus is very small - it has approximately one-sixteenth the mass of the oxygen atom. As a result, the net positive charge on the hydrogen atom has a relatively high charge density.
When two water molecules are sufficiently close together, one of the positively charged hydrogen atoms belonging to one of the water molecules (i.e. the proton donor) is strongly attracted to the negatively charged oxygen atom belonging to the other water molecule (the proton acceptor).
Hydrogen bonds are not as strong as ionic or covalent bonds, but they are much stronger than other types of secondary bond due to the hydrogen atom's high charge density, which increases the strength of the electrostatic attraction between the molecules involved in the bond.
When a water molecule forms, two of its six valence electrons participate in covalent bonds with the two hydrogen atoms, each of which can form hydrogen bonds with other molecules. This leaves the oxygen atom with two free pairs of electrons, both of which can also participate in hydrogen bonds with other molecules. A single water molecule can thus form hydrogen bonds with up to four other molecules.
In a container full of water, each water molecule can form hydrogen bonds with up to four other water molecules as shown below. Because hydrogen bonds are significantly weaker than covalent bonds, however, they will continually break and reform. One estimate puts the average number of hydrogen bonds per water molecule at any given time at 3.59.
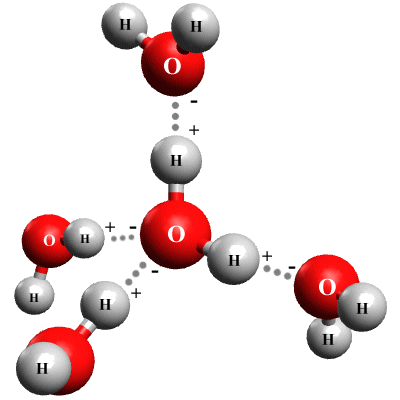
A water molecule can form hydrogen bonds with up to four other water molecules
The only elements whose atoms are sufficiently electronegative - and sufficiently small - to participate in a hydrogen bond are nitrogen, oxygen, and fluorine. Chlorine, for example, has an electronegativity that falls between that of nitrogen and that of oxygen, but because its larger size, it cannot form hydrogen bonds because its electron density is too low.
There are a number of intermediate kinds of chemical bonding that do not fall precisely into any of the categories discussed above. Covalent bonds, for example, involve two atoms sharing a pair of electrons, whereas in ionic bonds, a metal atom will relinquish one or more electrons to a non-metal atom.
Be aware however that a predomininantly ionic bond will often possess some covalent character because the electrons are still shared between the participating atoms. Conversely, a polar covalent bond in which there is separation of charge can also be said to be partially ionic in nature, because the shared electrons will spend more time around the nucleus of one atom than the other.
The degree to which a particular bond is ionic or covalent depends on the difference in electronegativity between the two atoms. Electronegativity is the degree to which an atom can pull electrons away from other atoms. The greater the difference in electronegativity, the more ionic the nature of the bond will be. Values are usually determined using the Pauling scale, named after the American chemist Linus Carl Pauling (1901-1994), who first proposed it in 1932, a dimensionless scale with a minimum value of 0.0 and a maximum value of 4.0.
A commonly used rule of thumb predicts that bonds created between the atoms of two different elements will be ionic in nature if the difference in electronegativity between them is 1.7 or greater; otherwise it will be covalent in nature. For example, the electronegativity value for sodium is 0.93, whereas the electronegativity value for chlorine is 3.16, giving a difference in electronegativity between these two elements of 2.23. This suggests that the chemical bonds formed between sodium and chlorine atoms will be strongly ionic in nature.
At the other end of the spectrum, in simple diatomic molecules such as O2 , there will be no difference in electronegativity, and hence no separation of charge, because both of the the participating atoms are of the same species (oxygen). The non-polar covalent bond in an O2 molecule is purely covalent and not even marginally ionic in character.
Most covalent bonds fall somewhere between these two extremes, depending on the degree of polarity involved. Polarity is a measure of the separation of charge, which depends on the relative electronegativities of the participating atoms. The greater the separation of charge, the stronger the ionic nature of the covalent bond will be.