| Author: | |
| Website: | |
| Page title: | |
| URL: | |
| Published: | |
| Last revised: | |
| Accessed: |
The properties of matter will to a large extent depend on what state the matter is in. Many familiar forms of matter have a particular state at room temperature, but make the transition to a different state when they become significantly hotter or colder. Examples of this phenomenon are all around us. Think about water, for example - something all living things need in order to survive (although those of us living in temperate climates often take it for granted).
At room temperature, water is a liquid. At temperatures of zero degrees Celsius (0 °C) or below, water starts to turn into ice, which is water in its solid state. At a temperature of one hundred degrees Celsius (100 °C) or above, water starts to turn into steam, which is water in its gaseous state (note that steam is actually invisible; what we often call steam is actually a mixture of steam and tiny water droplets that form as the steam condenses).
The process that matter goes through as it changes from one state to another is known as a phase transition. Phase transitions occur when sufficient energy is added to a system, or when sufficient energy is lost from a system. A chemical element or compound will undergo a phase transition at a specific temperature. The exact temperature at which the transition occurs will depend on pressure. For example, water boils at one hundred degrees Celsius (100 °C) at standard atmospheric pressure. At high altitudes, water will boil at a lower temperature because the atmospheric pressure is lower. The diagram below illustrates the different states of matter and the transitions that can occur between them.
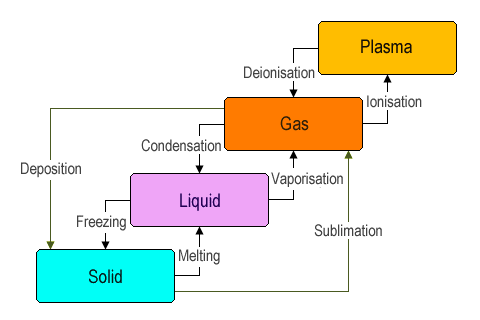
Transitions between the four common states of matter
As you can see, transitions occur in a specific order. At very low temperatures, the elementary entities (atoms, molecules or ions) making up a chemical element or compound do not have sufficient energy to overcome the bonds that hold them together. As a result, their movement is limited, and they tend to be packed together fairly densely to form rigid structures (i.e. solids). As temperature increases, these elementary entities acquire more energy.
The phase transition that occurs when a solid turns into a liquid is usually referred to as melting. As heat is applied to the solid, its elementary entities will vibrate faster as they acquire more energy. The bonds between the elementary entities weaken, allowing them to move around each other. The bonds between the elementary entities are still strong enough to keep them relatively close together, but they can no longer form rigid structures. A phase transition also occurs in the opposite direction when a liquid loses sufficient energy and becomes a solid. We call this process freezing.
If a liquid acquires enough energy, it can make the transition to a gas - a process known as vaporisation (due to either evaporation or boiling). This occurs when the liquid's elementary entities gain sufficient energy to overcome the forces holding them together. Once free of their bonds, these particles move around at high speed and in random directions; contact between them is limited to occasional collisions between the fast moving particles. A phase transition in the opposite direction occurs when a gas loses enough energy to revert to the liquid state - a process known as condensation.
When a substance changes state, its chemical composition stays the same, but its physical characteristics will alter significantly. A solid material tends to maintain its rigid structure because the there is not enough energy to overcome the forces holding its elementary entities together in a fixed position - which is why you can't poke your finger through a brick wall, for example. And, because the elementary entities are also in fairly close proximity to one another, a solid is difficult to compress.
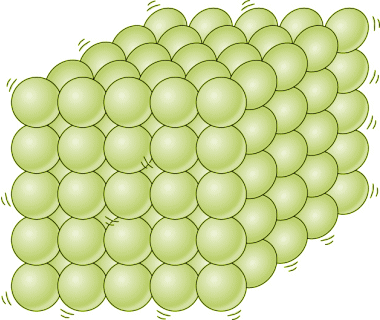
Elementary entities in a solid are tightly packed and are held in a fixed position
In the liquid state, the substance loses its ability to maintain a rigid shape because the elementary entities have gained enough energy to overcome the forces that hold them in place, and they can slide past one another. A liquid can thus adopt the shape of its container, and will be displaced by solid objects that are immersed in it - which is why you can put your hand in a bowl of water. Like solids, however, liquids, are difficult to compress because the elementary entities are still quite close together - you will feel significant resistance, for example, when wading through a stream.
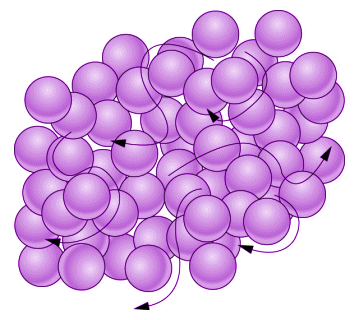
Elementary elements in a liquid can move around each other
In a gas, the elementary entities have gained sufficient energy to completely overcome the forces holding them together, and are able to move freely in any direction. Like a liquid, a gas will adopt the shape of its container. However, a gas differs from both a liquid and a solid in that its elementary entities will move apart from each other and occupy whatever space is available. Matter in the gaseous state is thus far less dense than matter in either the liquid or the solid state. One consequence of this is that a gas is far easier to compress than either a liquid or a solid. Another is that we can walk around in the Earth's atmosphere (which is made up of various gases) without encountering any significant resistance.
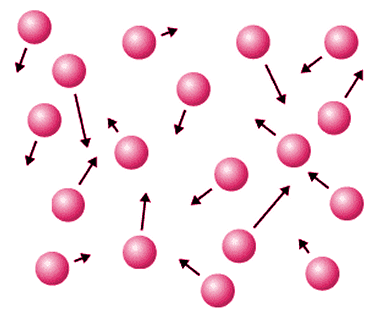
Gas particles can move freely and spread out to fill the available space
Something to note here is that, once the temperature of a solid sample has reached its melting point, any additional heat energy added to the sample will not increase the temperature of the sample further until the sample has completely melted. The same is true of a boiling liquid. The gas that is given off as the result of vaporisation will not increase in temperature until all of the liquid has boiled and turned into a gas.
Generally speaking, once a sample has attained its melting point or boiling point, adding more heat does not increase the temperature of the sample - it simply increases the speed at which the transition occurs. The reverse is also true as energy is lost. Once a sample of a gas has cooled down sufficiently to condense into a liquid, the temperature of the sample will remain constant until all of the gas has condensed. Similarly, when a sample of a liquid has reached its freezing point, the sample will not cool further until all of the liquid is frozen.
The fourth state of matter, as we mentioned in the introduction to this section, is plasma. The plasma state usually only occurs at very high temperatures. In this state, the elementary particles of matter become so energetic that electrons are able to break free of their atoms altogether - a process known as ionisation - resulting in the positively charged atomic nuclei (known as ions) floating around in a sea of negatively charged electrons. The reverse process to ionisation, in which energy is removed from the plasma so that electrons rejoin their atoms, is called deionisation. Because it consists of large numbers of positively and negatively charged particles, plasma will react far more strongly to electric and magnetic fields than matter in the other three states.

Stars like our Sun are essentially huge balls of plasma
You may have noticed from the diagram above that there are two further possible kinds of transition that we have not so far mentioned. It is possible, under the right circumstances, for solid matter to transition directly into a gas, skipping the liquid phase altogether. This process is known as sublimation. It can occur as the result of suddenly increasing the temperature of a solid beyond the boiling point of a substance (the substance is essentially vaporised), or when the substance is subject to very low pressure (i.e. when the environmental pressure surrounding the substance is equal to, or lower than, the vapour pressure of the substance (the topic of vapour pressure will be discussed elsewhere).
A gas can also transition directly into a solid - a process known as deposition. This can occur at high pressure, or when the temperature suddenly drops below the freezing point of a substance.
Although pressure undoubtedly has an influence on the point at which a phase transition will occur, by far the most important factor is energy. All matter consists of elementary entities (atoms, molecules or ions) that are constantly in motion. Even at temperatures approaching abslute zero, these elementary entities will posess some minimal amount of energy, and will vibrate.
The energy that an object possesses due to its motion is called kinetic energy, which is defined as the work that must be done on an object in order to make it accelerate from rest to its current velocity. We have seen that matter can only make the transition from one phase to another when it either acquires or relinquishes energy. When we heat a sample of a substance, we are in fact transferring kinetic energy to that substance, allowing its elementary entities to move faster. When we cool the same sample down, we are removing kinetic energy from the sample, causing its elementary entities to move more slowly.
The state in which matter exists is dependent on the amount of kinetic energy it possesses, which determines the degree to which its elementary entities can move in relation to one another. In solids, the elementary entities are locked in place and can do little more than vibrate. In liquids, they have enough energy to break free of their fixed positions and flow around each other. In gases, they have escaped the bonds that tie them to their neighbours altogether. And in plasma, there is enough kinetic energy to tear the electrons from their atoms to create a volatile and highly energised sea of charged particles.